After the IPO phase, we have the next phase’s tech challenges: life sciences clinical trials phase.
We know that sometimes, a life sciences organization may get to clinical trials before going public with an IPO. Regardless, in terms of the tech challenges of this phase, it’s common to see clinical trials become a highly manual process that organizations perform at the end of the month. It can typically take two to three days of a person’s time to manage this process, especially from a financial perspective.
Clinical trials bring audit concerns and require extensive communication, both with external vendors and internally with the project manager. The project manager needs to stay informed about the project’s status, progress, and completion. This almost always requires close collaboration between finance and business teams. Approval processes are also almost always in place for both purchasing and spending.
Lastly, the clinical trial phase involves significant legal documentation. Before finalizing a large purchase order with a CRO, the legal team must ensure that all necessary contracts are in place. This may involve integrating with third-party systems to manage the process effectively.
Clinical Trials Phase Common Systems
When managing clinical trials, we often observe that file storage and document management become increasingly complex at this stage. Changes to contracts are also becoming more common. To address this, many organizations adopt advanced systems like Agiloft or Conga to work in conjunction with NetSuite. These tools help manage the legal requirements, signoffs, change orders, and the complexities of document and storage management.
However, financial tracking of clinical trials and communication with vendors about progress remains the most cumbersome process. Sometimes, it’s as simple as asking if all invoices have been submitted, but other times, it involves more complex questions, like getting detailed updates on the trial’s progress and completion. Life sciences organizations typically handle all this communication—both simple and complex—through Excel spreadsheets and emails.
Clinical Trials Solved
During clinical trials, roles and responsibilities begin to shift. Initially, roles were more isolated, but now, this phase demands greater collaboration across functional teams. Scientists and operations need insight into budgeting, while finance depends on updates from scientists and project managers regarding completion percentages and project changes. Leadership, of course, has access to all contracts. It’s now essential for everyone to be able to report on and monitor the progress of trials from both financial and operational perspectives.
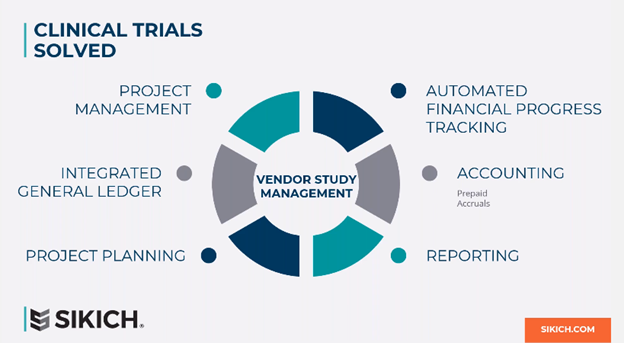
This is where we are going to introduce Sikich Success for Life Sciences, and how it solves clinical trials within NetSuite. At its most simplistic form, we have a concept of a project or vendor study and your dimensional chart of accounts and purchasing processes. On top of that, we give organizations the ability to establish monthly project plans. You can also track what you incurred versus what the vendor is invoicing you. Finally, you can then automate month- end journal entries as it relates to your prepaid or accruals around the clinical trials. That’s important, because when the audit comes around, NetSuite will be able to provide you with clear backups of your prepaid balance sheet accounts and positions.
After the clinical trials phase for life science organizations comes the end goal, the commercialization phase, which will be covered in the next post. If you have any questions about the clinical trials phase, we’re here to help. Please reach out to us at any time!
Other posts in this life sciences life cycle series:
This publication contains general information only and Sikich is not, by means of this publication, rendering accounting, business, financial, investment, legal, tax, or any other professional advice or services. This publication is not a substitute for such professional advice or services, nor should you use it as a basis for any decision, action or omission that may affect you or your business. Before making any decision, taking any action or omitting an action that may affect you or your business, you should consult a qualified professional advisor. In addition, this publication may contain certain content generated by an artificial intelligence (AI) language model. You acknowledge that Sikich shall not be responsible for any loss sustained by you or any person who relies on this publication.